The NIH has established a Respiratory Protection Program (RPP) in accordance with the Occupational Safety and Health Administration (OSHA) Respiratory Protection Standard (29 CFR 1910.134). The RPP provides NIH-wide procedures for the proper selection, use, and care of respiratory protective equipment, and is overseen by the Office of Research Services (ORS), Division of Occupational Health and Safety (DOHS). The RPP covers all NIH employees with the exception of those supporting clinical care in the NIH Clinical Center. The Clinical Center provides administrative management for respiratory protection of personnel working with pathogens of concern.
DOHS has multiple branches, two of which collaborate to facilitate the RPP: the Occupational Medical Service (OMS) and the Technical Assistance Branch (TAB). An RPP Manager is designated within TAB and assists the worksite manager with worksite evaluations, respirator selection, respirator training and respirator fit testing. OMS provides medical clearance to wear a respirator and enrolls employees in the Respiratory Protection Program.
Information for N-95 respirator and PAPR users
- When a work area has been evaluated and determined by a supervisor and safety representative that respiratory protection is required to protect NIH employees, those employees must be enrolled in the RPP. In this case, enrollment is required regardless of the type of respirator or PAPR the employee uses. The National Institutes of Health must approve the models of N95 respirators and PAPRs chosen. Appropriate respiratory protection PPE is provided to employees by the NIH at no cost to the employee.
Enrollment in the RPP must occur before using a respirator or any PAPR systems.
First time respirator and PAPR users must enroll in the program and complete the
Respiratory Initial Questionnaire in the MyCority database system. Users must re-submit the initial questionnaire every 3 years.
Once this step is complete, you will receive a confirmation email from OMS and Cority about your enrolment status and a recommendation to proceed to the following steps ( online training and fit test for N95 respirator users).
If you have submitted an initial questionnaire in the past 2 years you will need to complete the
Respiratory Periodic Questionnaire.
All RPP members are required to complete the following three steps annually:
- Complete
only one of the Medical Clearance Questionnaires ( Initial or Periodic) listed below . All forms are located in the New Questionnaire Folder of your MyCority profile:
https://orsp-cority1.ors.nih.gov/mycority/#/home or you can use one of the below links
If you never used the Cority system , the system would request that you create the offline pin. You can create an offline PIN or skip it by clicking on the Home page and then looking for the correct Respirator Questionnaire Form in the New Questionnaire File.

Always
Contact the OMS office with additional questions about your medical clearance forms or status.
The
Cority database and OMS will email you when your questionnaire is processed so you can proceed to the next step.
After the confirmation email,
N95 respirator users must follow the next two steps posted below. When you complete your N95 Respirator training, you will receive the booking link for the N95 fit test in the RPP fit test room. After completing the N95 fit test, your records will be posted in your Cority Profile.
PAPR users must complete only online training; their training records will be posted in their Cority Profile.
All your medical clearance and RPP records will be updated annually and can be reviewed by
employees and supervisors at any time.
Summary of the process:
|
Actions: |
Responsible department: |
STEP 1:This step must be completed annually according to your health and work period:
-
Respirator Initial Questionnaire- for new employees every 3 years or employees who have some drastic changes in their health conditions
-
Periodic Questionnaire – for employees who filled out the Initial form in the previous 3 years.
| OMS- Review and update your records in the Cority database system.
You will receive an official email from Cority and OMS about your enrollment status. |
Step 2:After the official OMS/ Cority email, follow the next step:
Complete the online training according to the respiratory PPE you will use in your work environment.
For example, medical personnel should complete the N95 training or MAXAIR PARR ( for employees with facial hair or medically assigned to it).
Research lab employees: Complete the N95 training, 3M Versaflo PAPR course, MAXAIR PAPR course, or all 3 courses because all PPE is present in your labs.
If you have any questions about the type of your PPE, please ask your supervisor or safety representative for your facility. Each facility has different respiratory hazards. | TAB- RPP- review your training records and update the Cority system with PAPR training records.
You will receive an official email from the TAB-RPP team about your quiz score. |
Step 3:After the N95 quiz, you will receive an email with your quiz score and booking link for the N95 respirator fit test.
Fit test is completed only by N95 respirator users.
PAPR users do not need to be fit-tested. | TAB-RPP- update the Cority system with the results of your fit test and model of the assigned N95 respirator.
You can find your records on the MyCority web page in a week. |
-
Complete respiratory protection training according to the PPE required for use in your work area.
-
Complete respirator fit testing. Fit testing is required annually thereafter. Fit testing is unnecessary for loose-fitting facepiece respirators, such as Powered Air-Purifying Respirators (PAPRs).
You will receive the link with the N95 fit test booking page after completing your N95 quiz.
For questions and additional information, email nihrespirator@mail.nih.gov.
Voluntary Use of a Respirator
Even when respirators are not required to be worn in a work area to protect employees, they can provide additional comfort and protection for employees. If an employee desires to wear a respirator on a voluntary basis, this must be discussed with their supervisor and the supervisor should request assistance from the RPP Manager if needed to determine that there are no airborne hazards that would require the use of a respirator.
Additionally, the use of the respirator itself must not present a health hazard to the wearer. To ensure this, some aspects of the RPP may need to be implemented, which will vary based on the type of respirator desired to be worn.
Note: Employees can be enrolled only in Respiratory Protection Program or Voluntary Respirator Use Program. Please, do not enroll your self in both programs.
The RPP team and OMS do not keep records for the Voluntary Respirator users.
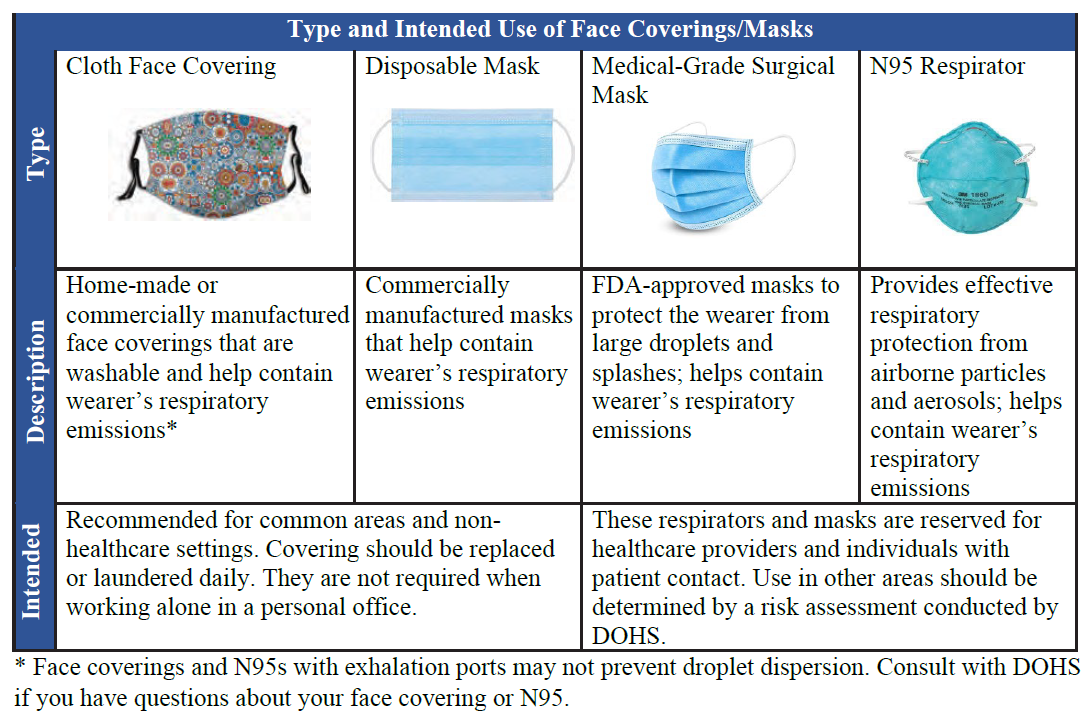
A filtering facepiece respirator (dust mask) is the most common respirator chosen to be worn voluntarily. Wearing a
NIOSH-approved filtering facepiece respirator (dust mask) is strongly recommended, and it will be labeled with a series of required markings. An "N95 respirator" is the most common particulate filtering facepiece respirator, which refers to the series (N; not resistant to oil) and the efficiency level (95; filters at least 95% of airborne particles). The performance of a dust mask which is not NIOSH-approved may vary, which is why they are not recommended for use, even voluntarily.
If after a discussion with the supervisor and the RPP Manager (if needed) and voluntary use of a filtering facepiece respirator (dust mask) has been permitted, employees must review the information contained in Appendix D of 29 CFR 1910.134, (Mandatory) Information for Employees Using Respirators When Not Required Under the Standard (see link below to review this information).
(NIH credentials required)
It is important to note that surgical masks do not meet the definition of a respirator. Still, it may be considered for wear based on a hazard analysis of the employee's work environment. For instance, in the context of respiratory infection control, surgical masks may be worn to protect a user from splashes of large droplets of blood or body fluids, or by an infected person to trap large particles of body fluids expelled by the wearer.
Coincidentally, there are combination products that are NIOSH-approved respirators and have also been cleared by the Food and Drug Administration as a surgical mask. These products are respirators, and so Appendix D information shall be provided to the user prior to voluntary use.
Voluntary use of any other respirator besides a filtering facepiece (dust mask) (e.g., an elastomeric half-mask with cartridge filters, or a full-face respirator) will require additional program elements. Contact the RPP Manager for assistance in making voluntary respirator use decisions and completing relevant program element requirements associated with their use.
Additional Information
Contact Information
If you have any questions regarding the RPP, please email nihrespirator@mail.nih.gov or call
(301) 496-2960 and ask for the RPP Manager.
back to the top
